Estudio dinámico de adsorción de Ni (II) sobre residuos de Musa aab simmonds
Dynamic study of NI (II) adsorption onto Musa aab simmonds residue.
Barra lateral del artículo
Términos de la licencia (VER)

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Declaración del copyright
Los autores ceden en exclusiva a la Universidad EIA, con facultad de cesión a terceros, todos los derechos de explotación que deriven de los trabajos que sean aceptados para su publicación en la Revista EIA, así como en cualquier producto derivados de la misma y, en particular, los de reproducción, distribución, comunicación pública (incluida la puesta a disposición interactiva) y transformación (incluidas la adaptación, la modificación y, en su caso, la traducción), para todas las modalidades de explotación (a título enunciativo y no limitativo: en formato papel, electrónico, on-line, soporte informático o audiovisual, así como en cualquier otro formato, incluso con finalidad promocional o publicitaria y/o para la realización de productos derivados), para un ámbito territorial mundial y para toda la duración legal de los derechos prevista en el vigente texto difundido de la Ley de Propiedad Intelectual. Esta cesión la realizarán los autores sin derecho a ningún tipo de remuneración o indemnización.
La autorización conferida a la Revista EIA estará vigente a partir de la fecha en que se incluye en el volumen y número respectivo en el Sistema Open Journal Systems de la Revista EIA, así como en las diferentes bases e índices de datos en que se encuentra indexada la publicación.
Todos los contenidos de la Revista EIA, están publicados bajo la Licencia Creative Commons Atribución-NoComercial-NoDerivativa 4.0 Internacional
Licencia
![]()
Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-NoDerivativa 4.0 Internacional
Contenido principal del artículo
Resumen
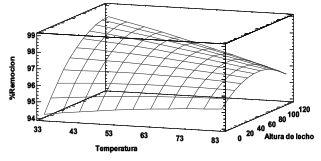
Se estudió la dinámica de adsorción de Ni (II) a partir de la torta residual del proceso de extracción de almidón de plátano en columna de lecho fijo variando la temperatura y altura de lecho. La biomasa se caracterizó por análisis elemental y FTIR. La concentración final del ion en solución se determinó por espectrofotometría de absorción atómica. Se encontró que los grupos funcionales hidroxilos y carboxilos son los de mayor protagonismo en la retención del ion. Del análisis ANOVA se determinó que las variables estudiadas en la remoción del Ni (II) no presentan efectos significativos sobre el mismo. De la curva de ruptura se encontró que la capacidad de adsorción máxima de la columna fue de 18.72 mg/g. El modelo de Dosis de Respuesta es el que mejor describe el proceso de adsorción, concluyendo que la torta residual utilizada es una alternativa de bajo costo muy eficiente en la remoción de Ni (II) a condiciones ambientales.
Descargas
Detalles del artículo
Candelaria Tejada-Tovar, Universidad de Cartagena
Ingeniera Química, Magister en Ingeniería Ambiental, Profesora Titular del programa del programa de Ingeniería Química, Facultad de Ingeniería, Universidad de Cartagena, Cartagena, Colombia, Process Design and Biomass Utilization Research Group (IDAB), Avenida del Consulado Calle 30 No. 48 – 152, Colombia; 130015
Referencias (VER)
Abbas, A.; Hussain, M. A.; Sher, M.; Irfan, M. I.; Tahir, M. N.; Tremel, W.; Hussain, S. Z.; & Hussain, I. (2017). Design, characterization and evaluation of hydroxyethylcellulose based novel regenerable supersorbent for heavy metal ions uptake and competitive adsorption. International Journal of Biological Macromolecules, 102, 170–180. https://doi.org/10.1016/j.ijbiomac.2017.04.024
Abdolali, A.; Ngo, H. H.; Guo, W.; Zhou, J. L.; Zhang, J.; Liang, S.; Chang, S. W.; Nguyen, D. D.; & Liu, Y. (2017). Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresource Technology. https://doi.org/10.1016/j.biortech.2017.01.016
Altino, H. O. N.; Costa, B. E. S.; & Da Cunha, R. N. (2017). Biosorption optimization of Ni(II) ions on Macauba (Acrocomia aculeata) oil extraction residue using fixed-bed column. Journal of Environmental Chemical Engineering, 5(5), 4895–4905. https://doi.org/10.1016/j.jece.2017.09.025
Azadi, F.; Saadat, S.; & Karimi-Jashni, A. (2018). Experimental Investigation and Modeling of Nickel Removal from Wastewater Using Modified Rice Husk in Continuous Reactor by Response Surface Methodology. Iranian Journal of Science and Technology - Transactions of Civil Engineering, 42(3), 315–323. https://doi.org/10.1007/s40996-017-0090-z
Azimi, A.; Azari, A.; Rezakazemi, M.; & Ansarpour, M. (2017). Removal of heavy metals from industrial wastewaters: A Review. ChemBioEng Reviews, 4(1), 37–59. https://doi.org/10.1002/cben.201600010
Barquilha, C. E. R.; Cossich, E. S.; Tavares, C. R. G.; & Silva, E. A. (2017). Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. Journal of Cleaner Production, 150, 58–64. https://doi.org/10.1016/j.jclepro.2017.02.199
Bibaj, E.; Lysigaki, K.; Nolan, J. W.; Seyedsalehi, M.; Deliyanni, E. A.; Mitropoulos, A. C.; & Kyzas, G. Z. (2019). Activated carbons from banana peels for the removal of nickel ions. International Journal of Environmental Science and Technology, 16(2), 667–680. https://doi.org/10.1007/s13762-018-1676-0
Boucherdoud, A.; Kherroub, D. E.; Bestani, B.; Benderdouche, N.; Douinat, O.; & History, A. (2021). Fixed-bed adsorption dynamics of methylene blue from aqueous solution using alginate-activated carbon composites adsorbents ARTICLE INFO ABSTRACT/RESUME. Algerian Journal of Environmental Science and Technology Month Edition, 0(0). www.aljest.org
Butler, L.; Lall, U.; & Bonnafous, L. (2017). Cumulative heavy metal contamination in mining areas of the Rimac, Peru basin (pp. 1–27). http://water.columbia.edu/files/2018/01/13.2017.Butler.Draft_.Cumulative-heavy-metal-contamination-in-mining-areas.pdf
Chao, H. P.; Chang, C. C.; & Nieva, A. (2014). Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. Journal of Industrial and Engineering Chemistry, 20(5), 3408–3414. https://doi.org/10.1016/j.jiec.2013.12.027
Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M. S.; & Catalano, A. (2020). Nickel: Human health and environmental toxicology. In International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph17030679
Gómez, V. E.; Herrera, A. P.; & Sánchez, J. H. (2019). Removal of acetylsalicylic acid (Asa) in packed microcolumns with carbon xerogel modified with TiO2 nanoparticles. Ingenieria e Investigacion, 39(2), 11–20. https://doi.org/https://doi.org/10.15446/ing.investig.v39n2.67604
Herrera-Barros, A.; Bitar-Castro, N.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; & González-Delgado, Á. D. (2020). Nickel adsorption from aqueous solution using lemon peel biomass chemically modified with TiO2 nanoparticles. Sustainable Chemistry and Pharmacy, 17, 100299. https://doi.org/10.1016/j.scp.2020.100299
Hokkanen, S.; Bhatnagar, A.; & Sillanpää, M. (2016). A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Research, 91, 156–173. https://doi.org/https://doi.org/10.1016/j.watres.2016.01.008
Jafari, S. A.; & Jamali, A. (2016). Continuous cadmium removal from aqueous solutions by seaweed in a packed-bed column under consecutive sorption-desorption cycles. Korean Journal of Chemical Engineering, 33(4), 1296–1304. https://doi.org/10.1007/s11814-015-0261-1
Li, W.; Yan, J.; Yan, Z.; Song, Y.; Jiao, W.; Qi, G.; & Liu, Y. (2018). Adsorption of phenol by activated carbon in rotating packed bed: Experiment and modeling. Applied Thermal Engineering, 142, 760–766. https://doi.org/10.1016/j.applthermaleng.2018.07.051
Liao, B.; Sun, W. yi; Guo, N.; Ding, S. lan; & Su, S. jun. (2016). Equilibriums and kinetics studies for adsorption of Ni(II) ion on chitosan and its triethylenetetramine derivative. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 501, 32–41. https://doi.org/10.1016/j.colsurfa.2016.04.043
Mahmood-Ul-Hassan, M.; Yasin, M.; Yousra, M.; Ahmad, R.; & Sarwar, S. (2018). Kinetics, isotherms, and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust. Environmental Science and Pollution Research, 25(13), 12570–12578. https://doi.org/10.1007/s11356-018-1300-3
Maniglia, B. C.; & Tapia-bl, D. R. (2016). Food Hydrocolloids Isolation and characterization of starch from babassu mesocarp. 55, 47–55. https://doi.org/https://doi.org/10.1016/j.foodhyd.2015.11.001
Martín-Lara, M. Á.; Trujillo Miranda, M. C.; Ronda, A.; Pérez Muñoz, A.; & Calero de Hoces, M. (2017). Valorization of olive stone as adsorbent of chromium(VI): comparison between laboratory- and pilot-scale fixed-bed columns. International Journal of Environmental Science and Technology, 14(12), 2661–2674. https://doi.org/10.1007/s13762-017-1345-8
Meneguin, J. G.; Moisés, M. P.; Karchiyappan, T.; Faria, S. H. B.; Gimenes, M. L.; de Barros, M. A. S. D.; & Venkatachalam, S. (2017). Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process Safety and Environmental Protection, 111, 244–252. https://doi.org/10.1016/j.psep.2017.07.005
Mishra, A.; Dutt, B.; & Kumar, A. (2016). Packed-bed column biosorption of chromium (VI) and nickel (II) onto Fenton modified Hydrilla verticillata dried biomass. Ecotoxicology and Environmental Safety, 132, 420–428. https://doi.org/10.1016/j.ecoenv.2016.06.026
Moino, B. P.; Costa, C. S. D.; da Silva, M. G. C.; & Vieira, M. G. A. (2017). Removal of nickel ions on residue of alginate extraction from Sargassum filipendula seaweed in packed bed. Canadian Journal of Chemical Engineering, 95(11), 2120–2128. https://doi.org/10.1002/cjce.22859
Moscatello, N.; Swayambhu, G.; Jones, C. H.; Xu, J.; Dai, N.; & Pfeifer, B. A. (2018). Continuous removal of copper, magnesium, and nickel from industrial wastewater utilizing the natural product yersiniabactin immobilized within a packed-bed column. Chemical Engineering Journal, 343, 173–179. https://doi.org/10.1016/j.cej.2018.02.093
Ratan, S.; Singh, I.; Sarkar, J.; & Rm, N. (2016). The Removal of Nickel from Waste Water by Modified Coconut Coir Pith. Chemical Sciences Journal, 7(3), 1–6. https://doi.org/10.4172/2150-3494.1000136
Romero-Cano, L. A.; García-Rosero, H.; Gonzalez-Gutierrez, L. V.; Baldenegro-Pérez, L. A.; & Carrasco-Marín, F. (2017). Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2017.06.032
Saadat, S.; Hekmatzadeh, A. A.; & Karimi Jashni, A. (2016). Mathematical modeling of the Ni(II) removal from aqueous solutions onto pre-treated rice husk in fixed-bed columns: a comparison. Desalination and Water Treatment, 57(36), 16907–16918. https://doi.org/10.1080/19443994.2015.1087877
Singh, S.; & Shukla, S. R. (2017). Theoretical studies on adsorption of Ni(II) from aqueous solution using Citrus limetta peels. Environmental Progress and Sustainable Energy. https://doi.org/10.1002/ep.12526
Sivarajasekar, N.; Mohanraj, N.; Baskar, R.; & Sivamani, S. (2018). Fixed-Bed Adsorption of Ranitidine Hydrochloride Onto Microwave Assisted—Activated Aegle marmelos Correa Fruit Shell: Statistical Optimization and Breakthrough Modelling. Arabian Journal for Science and Engineering, 43(5), 2205–2215. https://doi.org/10.1007/s13369-017-2565-4
Šoštarić, T. D.; Petrović, M. S.; Pastor, F. T.; Lončarević, D. R.; Petrović, J. T.; Milojković, J. V.; & Stojanović, M. D. (2018). Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. Journal of Molecular Liquids, 259, 340–349. https://doi.org/10.1016/j.molliq.2018.03.055
Sreenivas, K. M.; Inarkar, M. B.; Gokhale, S. V.; & Lele, S. S. (2014). Re-utilization of ash gourd (Benincasa hispida) peel waste for chromium (VI) biosorption: Equilibrium and column studies. Journal of Environmental Chemical Engineering, 2(1), 455–462. https://doi.org/10.1016/j.jece.2014.01.017
Šuránek, M.; Melichová, Z.; Kureková, V.; Kljajević, L.; & Nenadović, S. (2021). Removal of Nickel from Aqueous Solutions by Natural Bentonites from Slovakia. Materials, 14(2), 282. https://doi.org/10.3390/ma14020282
Tejada-Tovar, C.; Gallo-Mercado, J.; Moscote, J.; Villabona-Ortíz, A.; & Acevedo-Correra, D. (2018). Competitive adsorption of lead and nickel ont yam husk and palm bagasse in continuous system. Revista Biotecnología En El Sector Agropecuario y Agroindustrial, 16(1), 52–61. https://doi.org/http://dx.doi.org/10.18684/bsaa.v16n1.624
Tejada-Tovar, C. N.; Villabona-Ortíz, A.; & Ortega-Toro, R. (2020). Cr(VI) biosorption: Effect of temperature,particle size and bed height. Revista Facultad de Ingenieria, 96, 78–86. https://doi.org/10.17533/udea.redin.20191149
Valencia, J. A. R.; González, J. P.; Jimenez-Pitre, I.; & Molina-Bolívar, G. (2019). Physico-chemical treatment of waste water contaminated with heavy metals in the industry of metallic coatings. Journal of Water and Land Development, 43(1), 171–176. https://doi.org/10.2478/jwld-2019-0075
Villabona-Ortíz, A.; Tejada-Tovar, C.; González-Delgado, Á. D.; Herrera-Barros, A.; & Cantillo-Arroyo, G. (2019). Immobilization of Lead and Nickel Ions from Polluted Yam Peels Biomass Using Cement-Based Solidification/Stabilization Technique. International Journal of Chemical Engineering, 2019. https://doi.org/https://doi.org/10.1155/2019/5413960

 pdf
pdf
 FLIP
FLIP